Hydrogen has the potential to play a vital role in the clean energy transition. Due to its versatility, hydrogen can help tackle critical energy challenges and meet midcentury climate goals. It can reduce emissions in areas where other solutions like electrification, energy efficiency, or renewables are infeasible. This post will explain basic facts about hydrogen, including its important role in decarbonization and different production methods.
Key takeaways
- Hydrogen is a clean energy solution to help decarbonize critical sectors of the economy such as transportation, power generation, and manufacturing.
- Hydrogen’s versatility as an energy carrier distinguishes its long-term potential to contribute to meeting midcentury climate goals.
- Hydrogen production is flexible, with some production pathways capable of achieving low, zero, or net-negative emissions.
What is hydrogen?
Hydrogen is a colorless, odorless gas that does not produce greenhouse gases when burned making it poised to play a substantial role in decarbonization. Hydrogen is an energy carrier, meaning it can deliver and store energy from other sources. This quality makes it an ideal alternative to burning fossil fuels such as coal or methane (also known as natural gas).
Hydrogen can be produced from a variety of resources, such as natural gas, nuclear power, biogas, and renewable power like solar and wind. While hydrogen releases no emissions when burned as fuel, hydrogen production methods have varying emissions intensity levels.
What makes hydrogen clean?
The production pathway—how hydrogen is made—is what determines whether it is “clean” or not. Clean, in this context, refers to whether the production pathway results in significantly large amount of greenhouse gas emissions compared to other methods. While colors (e.g., blue hydrogen) are used to describe the variety of production pathways available for hydrogen, they are not directly correlated to how clean the method of production is.
Each of hydrogen’s production stages and end-uses needs to be measured to determine its total greenhouse gas emissions. This evaluation is often called a lifecycle greenhouse gas analysis and can help ensure that the production method creates low- or zero-carbon hydrogen across pathways. Accounting methods like this one are critical to ensuring that carbon dioxide (CO2) emissions are not overlooked during the production process and the eventual use of hydrogen.
The recently enacted Bipartisan Infrastructure Law provides a consistent measure of evaluating how “clean” hydrogen is. Hydrogen is considered “clean” for the purposes of funding opportunities if the entire production process creates two kilograms or less of CO2 for every kilogram of hydrogen produced.
How can hydrogen play an important role in decarbonizing our economy?
Hydrogen has three key advantages that distinguish it from other zero-carbon energy sources:
- It generates high heat when burned, resulting in zero-carbon emissions.
- It is suitable for seasonal energy storage, improving grid reliability.
- It can be flexibly produced with available resources, supporting an affordable and scalable clean energy transition.
Hydrogen can be used as a feedstock, fuel, and long-term energy storage to contribute to the decarbonization of current energy systems. Most hydrogen is used by the chemical, petroleum refining, and ammonia fertilizer industries. In the future, as hydrogen production diversifies, it has the potential to meet various needs in our economy. This potential includes uses for three of the most energy-intensive sectors: transportation, electricity generation, and manufacturing.
Transportation sector
For transportation, hydrogen can power zero-emissions vehicles through fuel cell technology. Unlike a traditional internal combustion engine used in most cars today, fuel cell vehicles use hydrogen and oxygen to emit water as exhaust, producing no CO2, particulate, or sulfur emissions.
Power sector
Hydrogen is an attractive option for decarbonizing the electric power sector because it allows the use of existing assets in the energy transition, avoiding the high capital costs of new development. In some cases, it can be blended with natural gas and distributed through existing natural gas transport infrastructure.
Hydrogen can also play a key role in enabling electric grid stability. It is particularly suitable for long-duration storage (meaning ten or more hours in this context). When energy demand is low and electricity generation is high, hydrogen can conserve electricity until demand increases. This quality is particularly useful as more seasonal and variable generation, such as renewable generation, increases. Hydrogen can offer the complementary capacity to efficiently store excess energy when energy production is high and exceeds demand.
Manufacturing and industrial sectors
Hydrogen can be used in the manufacturing and industrial sectors for either power generation or industrial applications. Notably, hydrogen can reach the high temperatures required for industrial heating processes, such as cement and steel manufacturing. For example, high heat is needed to chemically modify the ingredients for cement production. Reaching such high temperatures can be hard to achieve through other zero- and low-carbon strategies, such as electrification. Hydrogen can also serve as a feedstock – fuel material – for many chemical processes inherent to industrial production.
How is hydrogen produced?
Just as there are many opportunities to utilize hydrogen, there are also many production methods. While hydrogen is colorless, colors are often used to indicate how it is produced. Each production pathway uses different inputs to produce hydrogen, with a wide range of carbon emission-reduction options within even a single production method.
Flexible production allows for the best use of surrounding resources. For instance, in areas with an abundance of natural gas production, methane reformers can be used to produce hydrogen. The carbon emissions from those facilities can be captured and used or stored, resulting in low-carbon hydrogen. Electrolyzer plants offer opportunities in areas with abundant renewable and zero-carbon energy. And, areas with biomass waste feedstocks can combine gasification with carbon capture to produce hydrogen. That combination has the potential to produce hydrogen with net-negative emissions.
Although some production methods are more common, there are many ways to produce hydrogen including electrolysis, steam-methane and autothermal reforming, methane pyrolysis, and coal gasification.
Electrolysis
Hydrogen can be produced by using water electrolysis, which uses electricity to split water into oxygen and hydrogen gas. The potential for emissions reductions from electrolysis is dependent on the electricity source. This method is known as green hydrogen when the electricity is generated from renewable sources, such as solar, wind, geothermal, hydropower and ocean resources, or plant biomass.
Here’s what electrolysis looks like:
H2O + electricity = H2 + O
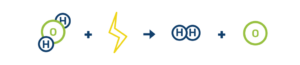
Note: H2O refers to water, H2 refers to hydrogen, and O refers to oxygen.
Hydrogen can also be made from nuclear power through electrolysis, with the methods often described in three colors:
- Pink hydrogen is produced when the electricity for electrolysis is sourced from a nuclear power plant.
- Purple hydrogen is produced when the electricity is produced using nuclear power and heat through combined chemo thermal electrolysis.
- Red hydrogen is produced through high-temperature catalytic splitting of water using nuclear power thermal as an energy source.
Steam-methane and autothermal reforming
Hydrogen can also be produced when natural gas is split into hydrogen and CO2 using steam-methane reforming or autothermal reforming. During steam-methane reforming, methane and water are heated and react to create carbon monoxide and hydrogen. Water is added to the system, reacting with the carbon monoxide to form CO2 and hydrogen.
Here’s what the steam-methane reforming chemical reaction looks like:
Step 1: Steam-methane reforming reaction
H2O + CH4 + (heat) → 3H2 + CO
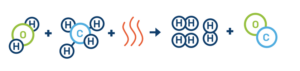
Note: CH4 refers to methane, CO refers to carbon monoxide
Step 2: Water-gas shift reaction
CO + H2O → CO2 + H2 + (small amount of heat)

Autothermal reforming uses oxygen and CO2 or steam in a reaction with methane to form synthesis gas. Synthesis gas is a fuel gas mixture consisting primarily of hydrogen, carbon monoxide, and very often CO2.
Here’s what the autothermal reforming reaction looks like:
4CH4 + O2 + 2H2O → 10H2 + 4CO
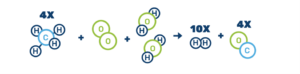
The carbon oxides created from both processes, steam-methane reforming or autothermal reforming, can be captured and utilized or stored to create a zero- or low-carbon production pathway:
- Blue hydrogen typically refers to hydrogen produced using steam-methane reforming and autothermal reforming where the carbon is captured and stored or utilized.
- Gray hydrogen commonly refers to hydrogen produced using steam-methane reforming or autothermal reforming where the carbon is not captured.
Other production types
There is a host of other production pathways you might encounter in the realm of hydrogen.
Some other production pathways include:
- Methane pyrolysis produces hydrogen through the thermal splitting of methane. This process is still experimental and removes the carbon in a solid form instead of CO2 gas. Hydrogen produced using methane pyrolysis is often called turquoise hydrogen.
- Coal gasification uses steam and oxygen to break molecular bonds in coal and form a gaseous mixture of hydrogen and CO2. Hydrogen created through this process is often called black or brown hydrogen.
- Naturally occurring hydrogen is often referred to as white hydrogen.
What’s next for hydrogen?
The Federal Government has recognized the importance of reaching cost-effective hydrogen production by 2030 and has awarded $9.5 billion through the Bipartisan Infrastructure Law to support clean hydrogen deployment. Eight billion dollars of that funding will go towards developing areas across the nation where hydrogen producers and consumers are closely located and connected. These areas are called hydrogen hubs. The Great Plains Institute’s Hydrogen and Carbon Hub Atlas explains what states and regions might have optimal resources and infrastructure to support hydrogen hubs.
This new funding means that the current situation for US hydrogen deployment is constantly changing. The first blog in the series, Hydrogen Hubs: The State of Play, provides an overview of federal funding for hydrogen hubs and explains how states and regions are planning to take advantage of near-term federal funding opportunities. The next blog in the series will cover the importance of hubs for the economywide deployment of hydrogen.
This blog was originally published by Great Plains Institute.
